About TMS
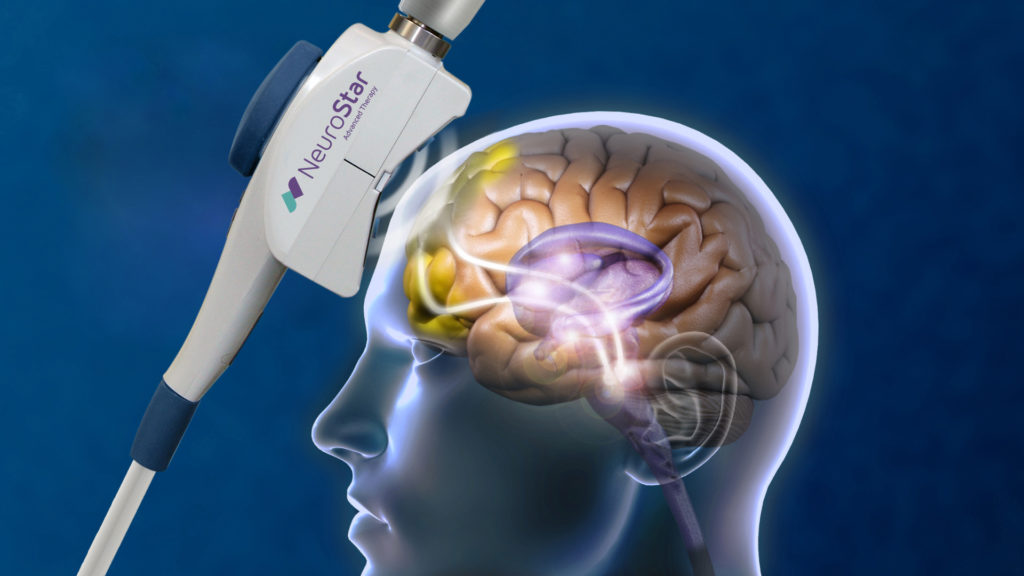
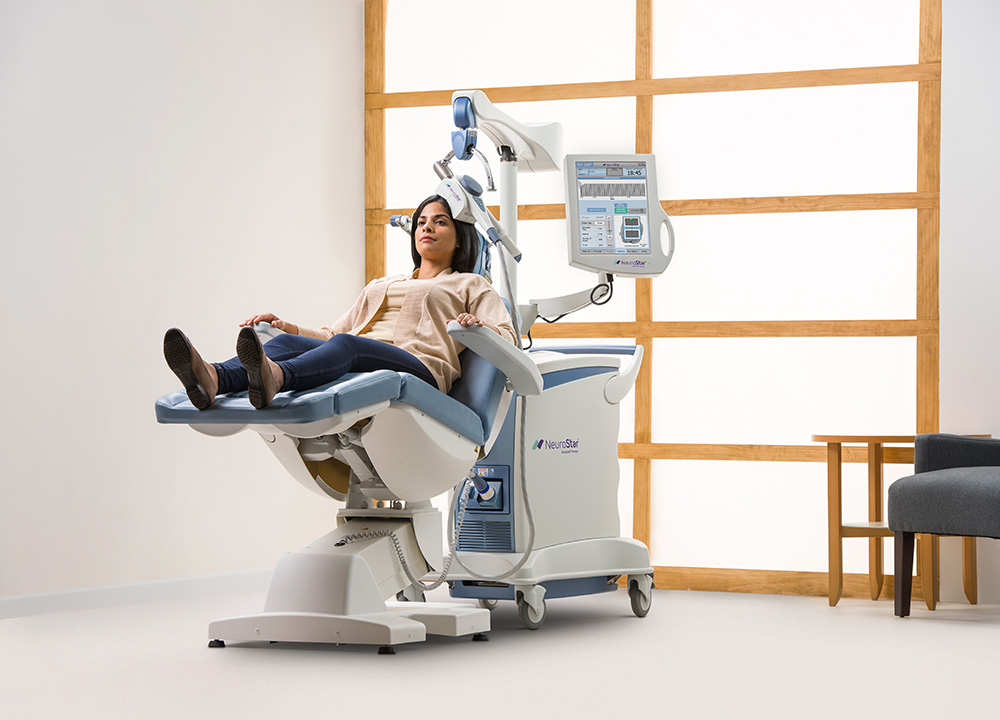
TMS (transcranial magnetic stimulation) is a non-invasive treatment that is used for the treatment of major depression. During treatment, a coil is placed on the scalp that sends magnetic currents to the part of the brain that regulates emotions. It works equally and often better than anti-depressant medication alone. It has significantly less side effects than traditional medication used to treat depression. It is FDA approved for major depression in adults and there is peer reviewed, evidence based-based research to support its use for PTSD, OCD and adolescent depression.
4.5 million
4.5 million people in the United States who have depression don’t benefit from antidepressants.
In 2008
In 2008, TMS was approved by the FDA as an alternative treatment to traditional anti-depressant medicaion.
1.5 million
1.5 million TMS treatments have been delivered to 60,000 patients to date.
in 19 minutes
Treatment is delivered in 19 minutes.
Authorized by the US Food and Drug Administration in 2008, TMS is an outpatient treatment, administered without the need for anesthesia, that allows patients to return to normal activity directly following treatment. Side effects reported after treatment are minimal, with most patients only experiencing minor discomfort. Patients undergoing TMS are not required to participate in any additional medication regimen.
Who Can Benefit from TMS
TMS is recommended for patients who:
- Have tried various rounds of different medications and psychotherapy with no relief from their symptoms
- Wish to avoid the side effects of medication (such as sexual dysfunction and weight gain)
- Are planning to become pregnant
- Do not have a seizure disorder or a history of seizures
- Do not have any metal implant within the head
How effective is TMS treatment?
30 clinical trials with 2,000 patients showed TMS to be effective for treating depression
In a real world naturalistic study, 58% of patients treated with TMS experienced significant improvement1
37% of people treated with TMS found relief from their depression symptoms2
People treated with TMS were 2-4 times more likely to achieve remission from depression symptoms, compared to people treated with antidepressants alone3
62.5% of people treated with TMS who experienced relief continued to show improvement after 1 year4
Footnotes
1- Carpenter LL et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-96.
2- O’Reardon JP et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry. 2007; 62(11):1208-16.
3- George MS et al. Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder.Arch Gen Psychiatry. 2010;67(5):507-16.
4- Dunner DL et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-401.
Myths and Facts
TMS is not the same as ECT (electroconvulsive therapy, formerly called “electroshock therapy” or “shock treatment”). While both therapies are used to treat major depression, ECT is usually administered in a hospital with anesthesia, which adds to the risk and recovery time of the procedure. Most physicians recommend ECT as an emergency measure because it is invasive and can lead to serious side effects, like memory loss. TMS is a non-invasive outpatient procedure that has demonstrated few to no side effects.
The safety and effectiveness of TMS has been supported by over 30 medical studies and multiple meta-analyses. It was approved by the FDA in October of 2008 and is widely considered a very low-risk procedure by the medical community as TMS does not require anesthesia and can be performed as an outpatient procedure.
Most patients only report minor discomfort as a side effect and can continue their life as usual directly following each procedure.
Other reported side effects of TMS treatment include:
- Difficulty sleeping
- Eye pain
- Facial pain/toothache
- Muscle twitch
In very rare cases (0.1%), patients may experience seizures after treatment. But it is important to note that no adverse cognitive effects, including memory loss, have been reported following TMS.
Patients and caregivers should be vigilant of behavioral changes during treatment — especially if they notice an increase in suicidal thoughts or behavior, or depression symptoms worsen.
Overall, the side effects of TMS treatment are few and low in severity when compared to the commonly-reported side effects of antidepressant medications. TMS provides a safe and effective alternative to these treatment methods.
TMS is recommended for patients who have tried many other therapies – including multiple medications and psychotherapy – with no relief. One study demonstrated an improvement in symptoms in 58% of participants, and a 37% full remission rate in individuals diagnosed with treatment-resistant depression who underwent TMS.